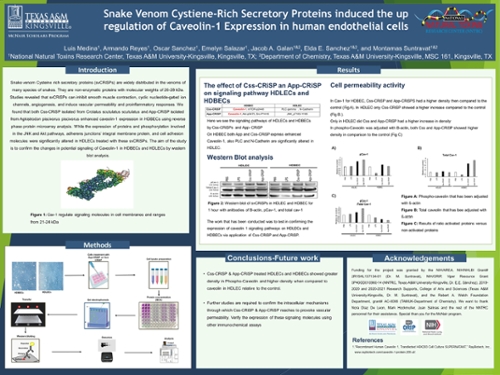
Snake venom Cysteine rich secretory proteins (svCRiSPs) are widely distributed in the venoms of many species of snakes. svCRiSPs are a member of a large protein family characterized by 16 conserved cysteine residues within two conserved domains, pathogenesis- related proteins of group 1 (PR-1) and a cysteine-rich domain (CRD). They are non-enzymatic proteins with molecular weights of 26-28 kDa. Studies revealed that svCRiSPs can inhibit smooth muscle contraction, cyclic nucleotide-gated ion channels, angiogenesis, and induce vascular permeability and proinflammatory responses. We initially screened the changes in protein expression and phosphorylation in human dermal lymphatic (HDLECs) and blood (HDBECs) endothelial cells after treatment with our two newly discovered svCRiSPs, Css- CRiSP isolated from the venom of Crotalus scutulatus scutulatus and App-CRiSP isolated from Agkistrodon piscivorus piscivorus, using reverse phase protein microarray analysis (RPPA). We found that both Css-CRiSP and App-CRiSP enhanced caveolin-1 expression in HDBECs. While the expression of proteins and phosphorylation involved in the JNK and Akt pathways, adherens junctions integral membrane protein, and cell adhesion molecules were significantly altered in HDLECs treated with these svCRiSPs. The aim of the study is to confirm the changes in potential signaling in HDBECs and HDLECs by western blot analysis. It has been shown that the expression of caveolin-1 is detected by using western blot image analysis.
Faculty Mentor: Dr. Montamas Suntravat
National Natural Toxins Research Center